0047 Let me go through the previous argument using the ongoing example.
Here is the science side looking at its own reflection (in the mirror of philosophy… er… theology).
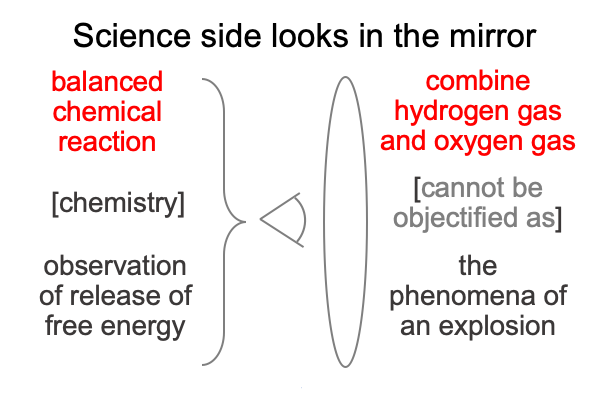
First, when a scientist looks into Tabaczek’s mirror, he covers the noumenon with the illumination of an appropriate model. Here, the noumenon is simply a recapitulation of the model of a balanced chemical reaction. This is typical for triumphalist… er… college-level laboratory science classes. If hydrogen and oxygen are combined in the laboratory, the phenomenon is an explosion. The explosion does not objectify the chemical reaction. Rather, the phenomenon is an observable and measurable facet of the chemical reaction.
I suppose that, in theory, hydrogen and oxygen gases can react without producing an explosion.
Hmmm…
Perhaps, the scientist has an insight. The explosion merely is a manifestation of the release of free energy when the reagents react. Perhaps, if a technologist alters conditions, some of that free energy can be extracted and channeled for some technical purpose.
0048 Here is a picture of the natural philosopher looking into the science side of the mirror.
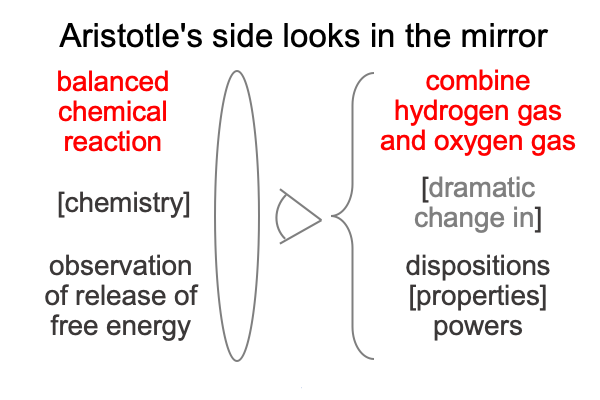
0049 The Aristotelian starts with the thing itself, hydrogen and oxygen as matter [substantiates] form. Already, matter reflects scientific knowledge, insofar as many natural philosophers will not ignore the widely accepted chemical principles of atoms and molecules. Hydrogen gas consists of two atoms of hydrogen sharing two electrons. Oxygen consists two atoms of oxygen holding sixteen electrons total.
Okay, the forms also reflect chemical principles, which brings me to Tabaczek’s stand-in for phenomena: dispositions [properties] powers. This hylomorphe is another way to express the character of hydrogen and oxygen molecules.
Hydrogen molecules (H2) have certain properties. They are disposed to give away their two electrons (-1), allowing their respective electropositive protons (+1) to find more accommodating clouds of electronegativity. Hydrogen molecules have the power to “reduce” other chemicals (where the “reduced” chemical gains electrons). “Reduction” means that a (not hydrogen molecule) chemical receives two electrons.
Oxygen molecules (O2) have certain properties. Each oxygen atom is disposed to take two electrons (-1) in order to better surround its electropositive nucleus (+8) with a complete second “shell” of electronegativity (resulting in nucleus at +8 and ten electrons adding to -10). Oxygen has the power to “oxidize” other chemicals (where the “oxidized chemical loses electrons). “Oxidation” means that a (not oxygen molecule) chemical loses four electrons (that is, two for each oxygen).
In order to explain these disposition [properties] powers, one may consider a hydrogen or an oxygen atom in terms of a point-sized electropositive nucleus inside standing waves or localized clouds of electronegative orbitals, each open to holding a pair of electrons. Each type of atom, each element, is composed of a point-sized electropositive nucleus of a certain mass and charge, surrounded by mathematical standing waves, which may (or may not) be occupied by one or two electrons. An atom’s tendency to give, cling to, share and take electrons depends of how each type of atom goes about getting the most stable… er… “stable” of electrons in its mathematical standing-wave “barn”.
Thus, the dispositions [properties] powers of molecular hydrogen and oxygen depend on the matters [substances] formsof their respective atoms.
So, if two molecular hydrogens (2H2 (g)) and one molecular oxygen (O2 (g)) are unhappy with their matters [substances] forms, then after an explosive release of energy, they settle in as two molecules of water (2H2O (g)) and their mathematically-defined standing-wave “barns” are happily occupied…. or… stabilized.
0050 Of course, this ranch-grown narrative depends on science, yet it sounds like natural philosophy, because it expresses what the scientist (or any person) encounters in terms of Peirce’s category of secondness. For all practical purposes, the noumenon offers a description of the laboratory-based balanced chemical reaction and its phenomena offers insights into the dispositions [properties] powers involved in spontaneous “orthograde” natural processes.
0051 Here is a general picture.
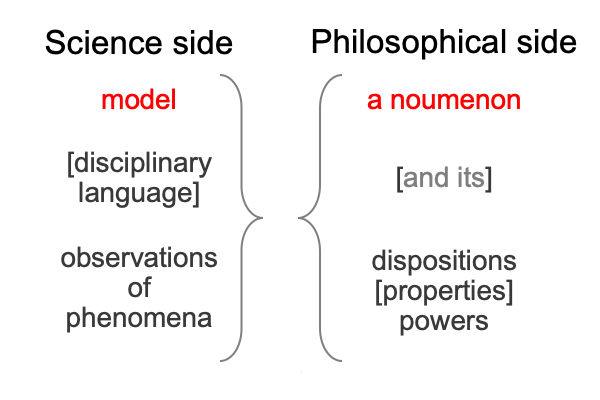
Needless to say, each side of the mirror projects into the other side of the mirror.
This constitutes Tabaczek’s challenge, now that the positivist intellect has been terminated.
The science side sees the ghost of the positivist intellect in its mirror.
The philosophical side sees science as it is practiced in its mirror.
